MDPC‑8127
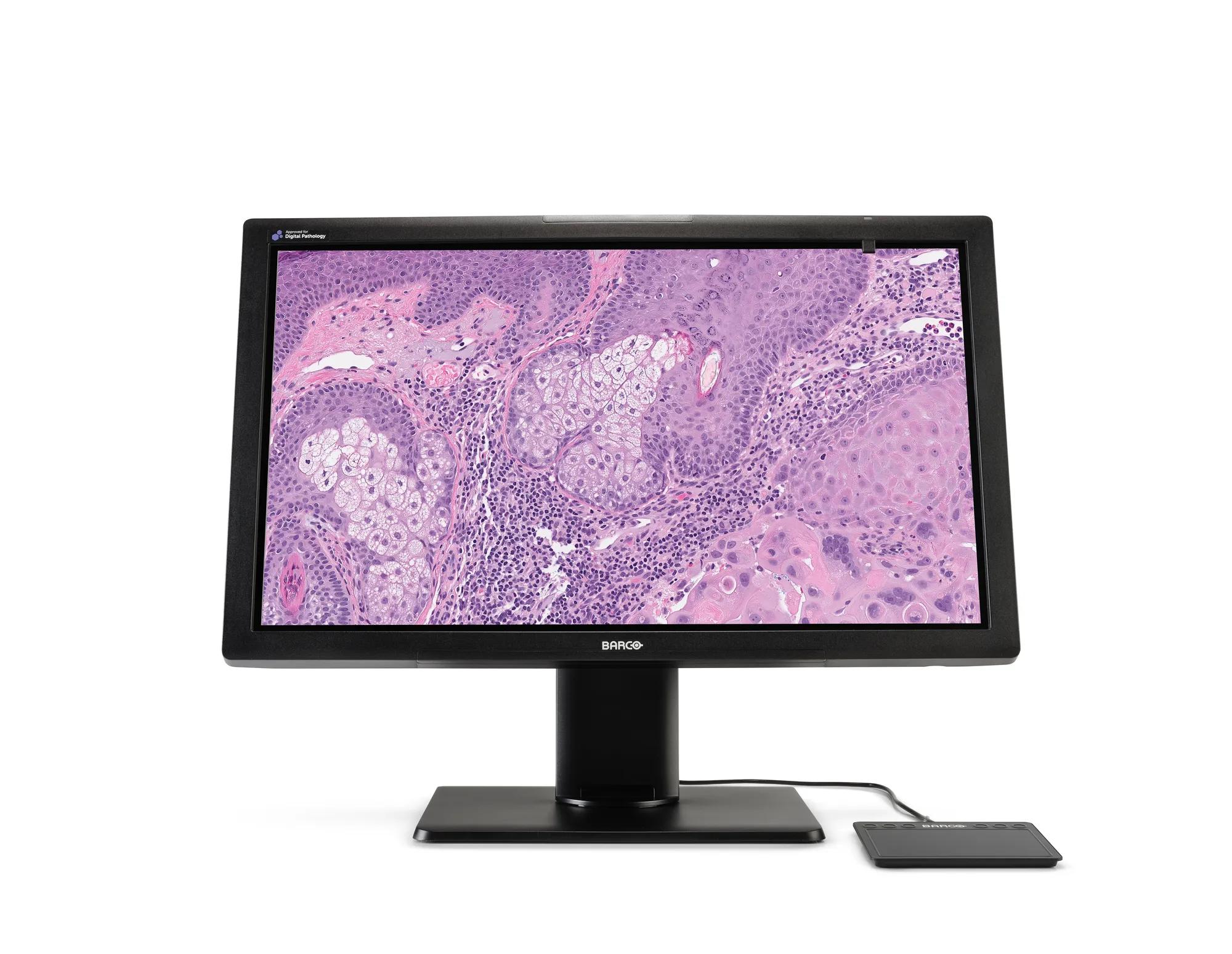
27" 8MP, ultra-high definition display, cleared for digital pathology
MDPC‑8127
27" 8MP, ultra-high definition display, cleared for digital pathology
- Visual richness and color confidence
- Panning and zooming with minimal blurring
- Stable image quality & automated calibration
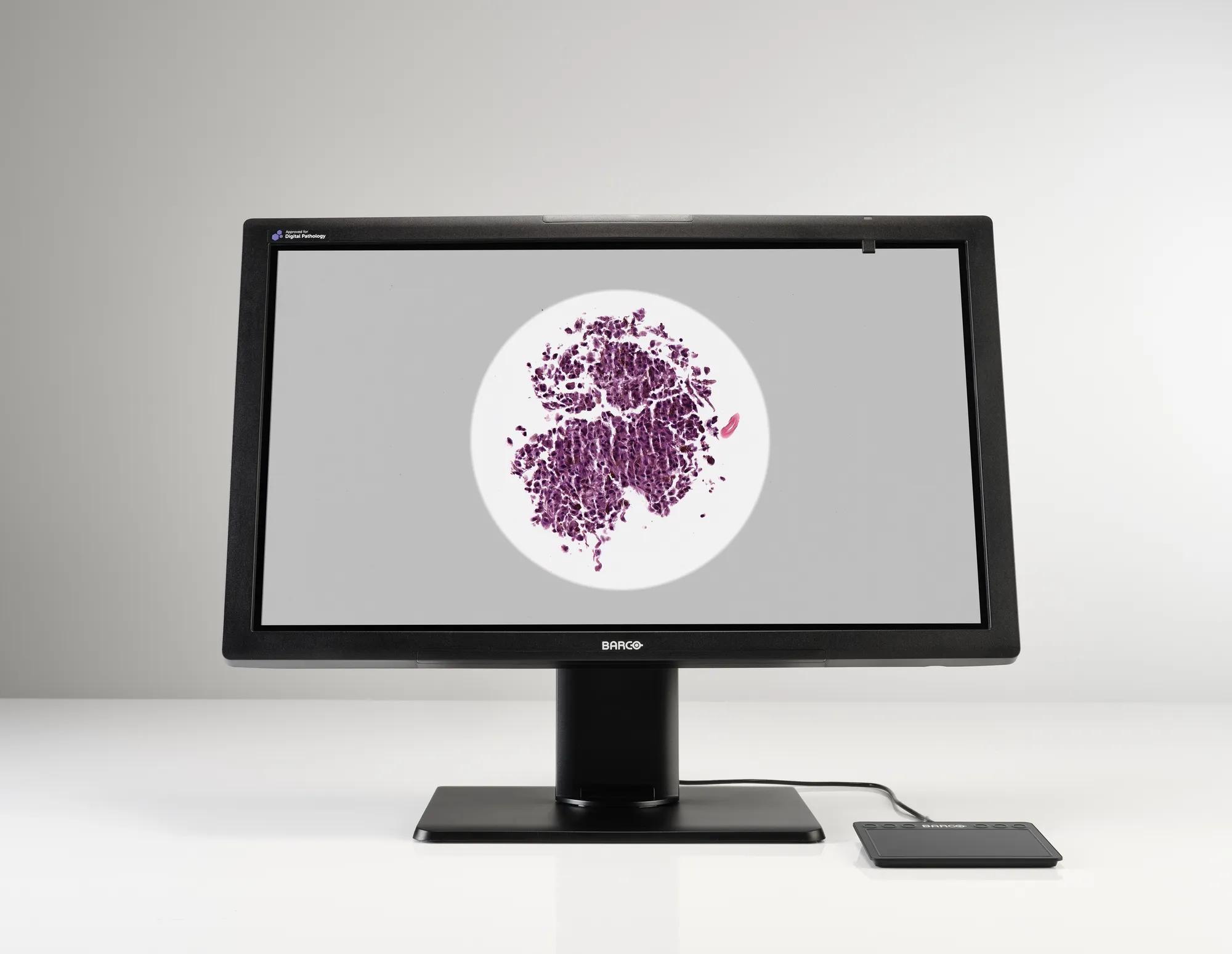
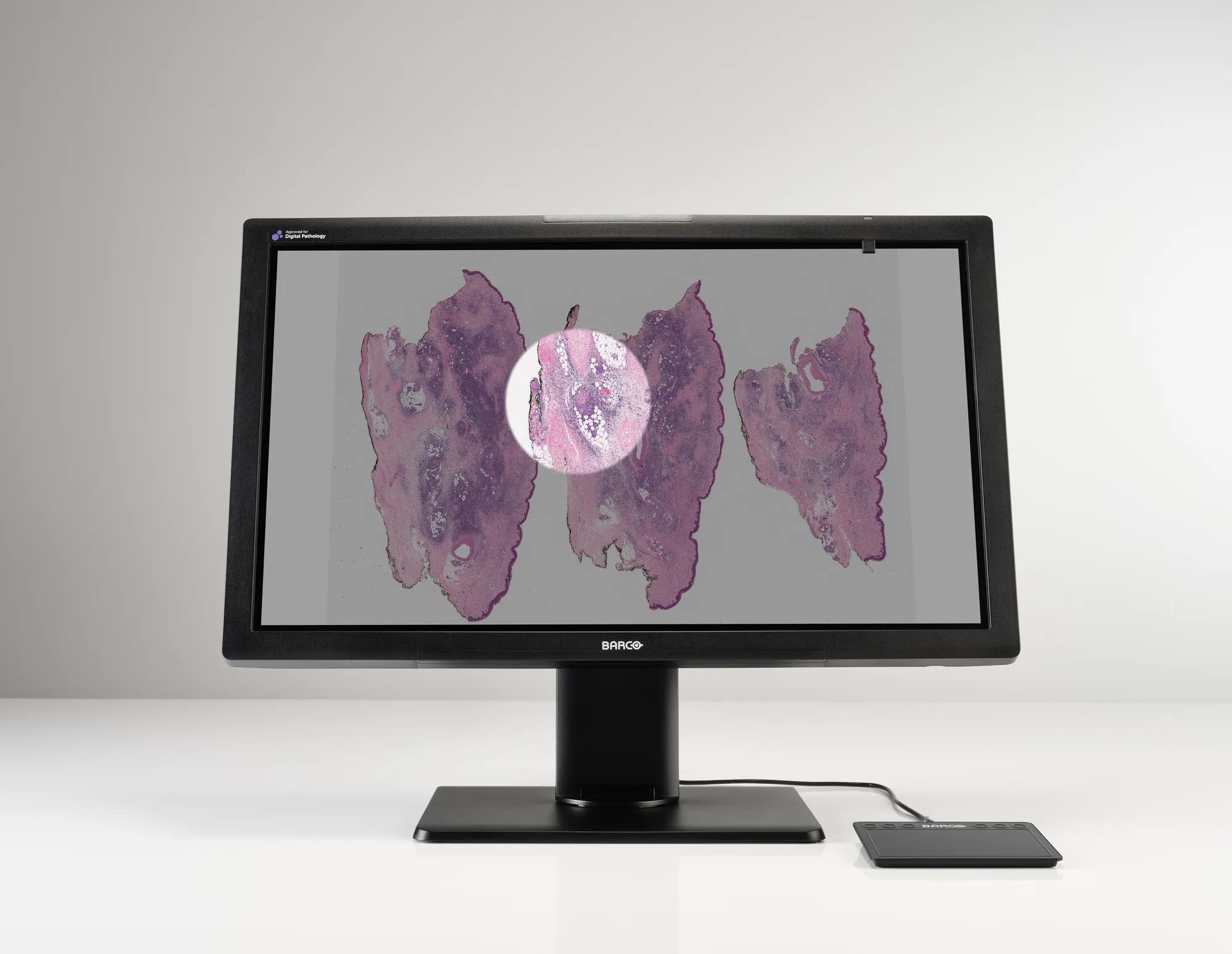
Meet MDPC-8127, our ultra-high definition medical grade display designed exclusively for digital pathology. With regulatory clearances for use in digital pathology including primary diagnosis, it’s the first display that you can confidently integrate into your digital pathology workflow with multiple whole slide imaging systems.* Work with superior imaging technology and analyze your histological samples confidently, with unprecedented visual richness.
*In the USA the MDPC-8127 can be used with WSI scanners and viewing software that have been validated for use with the display.
The device may be used for primary diagnosis in the following validated FDA-cleared WSI systems and digital pathology viewing software:
-
Philips Intellisite Pathology Solution with Philips Image Management System viewing software
-
Philips Intellisite Pathology Solution with Paige.AI Inc. FullFocus DX viewing software
-
Leica Aperio AT2 DX System with ImageScope DX viewing software
-
Leica Aperio AT2 DX scanner with Sectra Digital Pathology Module
-
Leica Aperio GT 450 DX scanner with Aperio WebViewer DX
-
Hamamatsu NanoZoomer S360MD slide scanner system
Specifications
Download spec sheetGeneral specifications
- Screen technology
- IPS LCD with LED backlight
- Active screen size (diagonal)
- 684 mm (27")
- Active screen size (H x V)
- 569 x 335 mm (22.4 x 13.2")
- Aspect ratio (H:V)
- 16:9
- Resolution
- 8MP (3840 x 2160 pixels @ 120 Hz)
- Pixel pitch
- 0.155 mm
- Color imaging
- Yes
- Gray imaging
- Yes
- Bit depth
- 10 bit (1.07 billion possible colors)
- Viewing angle (H, V)
- 178°
- Uniformity correction
- PPU
- SteadyColor
- Yes, with QAWeb Enterprise
- Color gamut NTSC
- 115% (typical)
- Color gamut sRGB
- 132% (typical)
- Color gamut DCI-P3
- 105% (typical)
- sRGB Delta E2000 (typical)
- < 1 (average)
< 3 (maximum)
- Ambient light presets
- Yes, reading room selection
- Ambient light sensor
- Yes
- Front sensor
- Yes, I-Guard
- Maximum luminance (panel typical)
- 850 cd/m²
- DICOM calibrated luminance
- 450 cd/m²
- Contrast ratio (panel typical)
- 1000:1
- Response time ((Tr + Tf)/2) (typical)
- 8 ms
- Housing color
- Black / White
- Video input signals
- 2x DisplayPort 1.2
- USB ports
- 1x USB 2.0 upstream (endpoint)
2x USB 2.0 downstream
- Power rating
- 100-240 Vac, 50/60 Hz, 3.6-1.6 A
- Power consumption
- 75 W (nominal) @ calibrated luminance of 450 cd/m²
< 0.5 W (hibernate)
< 0.5 W (standby)
- Dimensions with stand (W x H x D)
- 651 x 482~582 x 238 mm
- Dimensions w/o stand (W x H x D)
- 651 x 390 x 66 mm
- Dimensions packaged (W x H x D)
- 800 x 650 x 295 mm
- Net weight with stand
- 12.5 kg
- Net weight w/o stand
- 7.9 kg
- Net weight packaged
- 17.4 kg (without optional accessories)
- Tilt
- -5° to +25°
- Swivel
- -30° to +30°
- Pivot
- N/A
- Height adjustment range
- 100 mm
- Mounting standard
- VESA (100 mm)
- Screen protection
- Anti-Glare coating
- Recommended modalities
- Digital Pathology and Whole Slide Imaging
- Certifications
- CE0123 (Medical Device)
FDA 510(k) K203364
CCC (China)
Safety specific:
IEC 60950-1:2005 + A1:2009 + A2:2013
EN 60950-1:2006 + A1:2010 + A11:2009 + A12:2011 + A2:2013
IEC 60601-1:2005 + A1:2012
EN 60601-1:2006 + A1:2013 + A12:2014
ANSI/AAMI ES 60601-1:2005 + R1:2012
CAN/CSA C22.2 No. 60601-1:2014
EMI specific:
IEC 60601-1-2:2014 (ed.4)
EN 60601-1-2:2015 (ed.4)
FCC part 15 Class B
ICES-001 Level B
VCCI
Environmental:
China Energy Label, EU RoHS, China RoHS, REACH, Canada Health, WEEE, Packaging Directive
- Supplied accessories
- User guide
Documentation disc
Video cables
Mains cables
USB cable
Barco Touchpad
- Optional accessories
- MXRT display controller
- QA software
- QAWeb Enterprise
- Warranty
- 5 years, including 20000 hrs backlight warranty
- Operating temperature
- 0 °C to 35 °C (20 °C to 30 °C within specs)
- Storage temperature
- -20 °C to 60 °C
- Operating humidity
- 8% to 80% (non-condensing)
- Storage humidity
- 5% to 85% (non-condensing)
- Operating pressure
- 50 kPa minimum
- Storage pressure
- 50 to 106 kPa
You can now find all media, brochures, presentations, whitepapers & marketing downloads in our new & improved download center
Visit Media CenterLooking for technical documents or product support?
For technical downloads such as drivers, firmware, manuals, drawings & documentation we would kindly like to direct you to our product support page.
Go to product support