- Shows sharp, bright dental images
- Reveals more details
- A++ ecolabel thanks to high energy efficiency
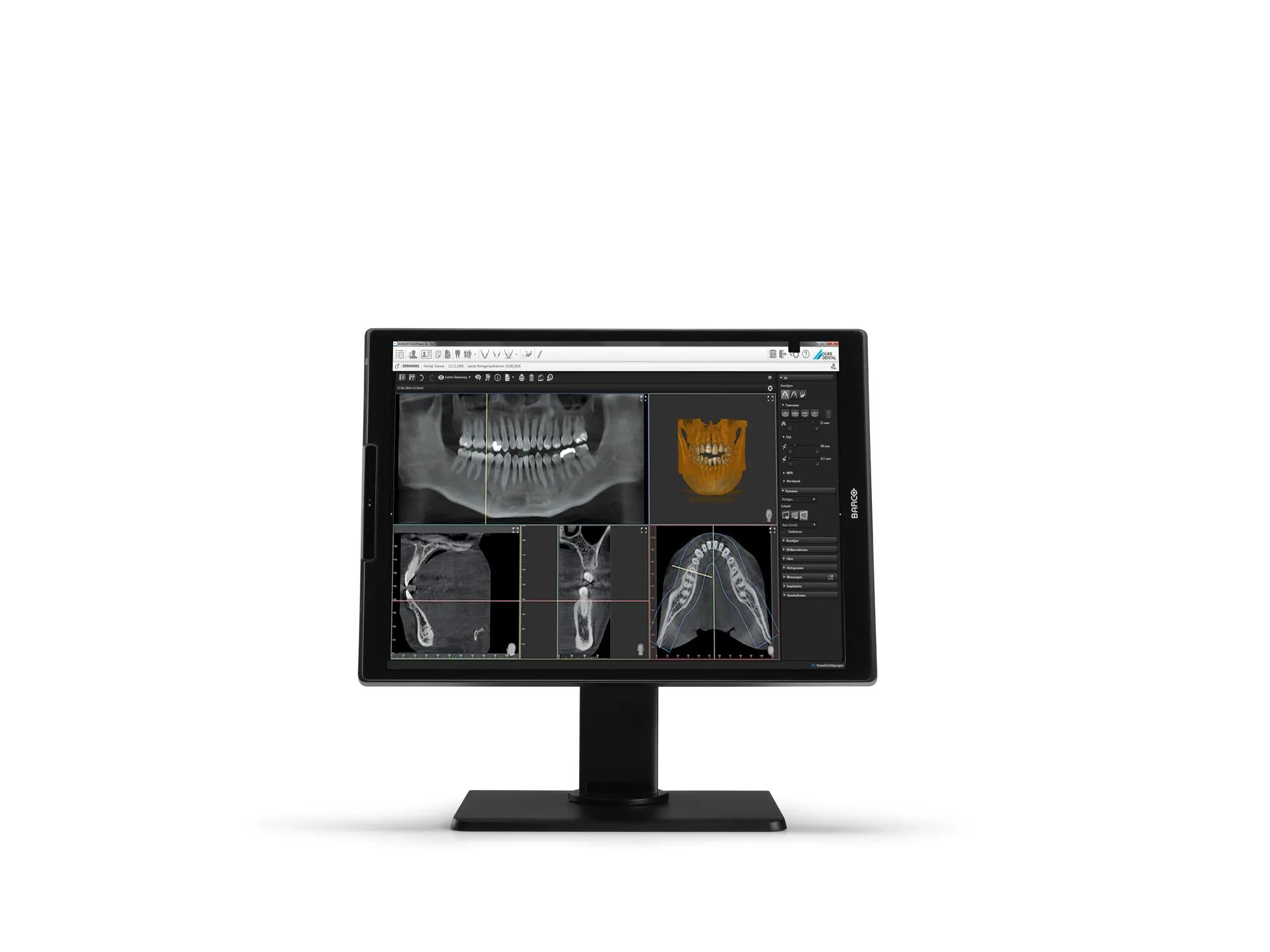
Our Nio Color 3MP dental display has everything it takes for dental specialists. It brings sharp and bright images to the diagnostic reading room so you can quickly visualize concealed anatomic structures and easily detect dental abnormalities. The Nio Color 3MP is available with and without cover.
An accurate diagnosis
The Nio Color 3MP dental display has been designed specifically for use in the dental reading room. For endodontic treatment, for example, visualization of small details is essential for a swift and correct diagnosis and an accurate treatment plan. Even when used in different ambient light conditions, the Nio Color 3MP display will provide you with crisp, high- contrast detail, enabling an accurate treatment planning.
Planning with confidence
Thanks to the integrated uniformity technologies, subtle abnormalities or concealed anatomical structures in the oral and maxillofacial region can be visualized without imperfections or screen noise. While keeping the brightness steady over time, you can make accurate decisions.
In the case of oral surgery, it means critical structures can be more visible, which is crucial when planning for dental implant placement, for example. Detailed visualization of the bone structure, accurate localization of nerves and arteries all contribute to the success and safety of the procedure.
Models
- Nio Color 3MP dental with protective cover
- Nio Color 3MP without cover
Please consult your Barco representative or distributor in your country or territory to confirm availability. A reference to any product or service on this site does not imply that such product is or will be available in your location.
Specifications
Download spec sheetGeneral specifications
- Screen technology
- IPS-SFT Color LCD
- Active screen size (diagonal)
- 541 mm (21.3")
- Active screen size (H x V)
- 433 x 325 mm (17.1 x 12.8")
- Aspect ratio (H:V)
- 4:3
- Resolution
- 3MP (2048 x 1536 pixels)
- Pixel pitch
- 0.2115 mm
- Color imaging
- Yes
- Gray imaging
- Yes
- Bit depth
- 30 bit
- Viewing angle (H, V)
- 178°
- Uniformity correction
- ULT
- SteadyGray
- Yes (in MXRT display controller), when used as a system with MXRT display controller & QAWeb Enterprise
- SteadyColor
- Yes (in MXRT display controller), when used as a system with MXRT display controller & QAWeb Enterprise
- RapidFrame
- Yes
- Ambient light presets
- Yes, reading room selection
- Ambient light sensor
- Yes
- Front sensor
- Yes, I-Guard
- Presence sensor
- Yes
- Maximum luminance (panel typical)
- 1050 cd/m²
- DICOM calibrated luminance
- 600 cd/m²
- Contrast ratio (panel typical)
- 2000:1
- Response time ((Tr + Tf)/2) (typical)
- 12 ms (gray-to-gray average)
- Housing color
- Black (RAL 9004) / White (RAL 9003)
- Video input signals
- 2x DisplayPort 1.4
- Video output signals
- N/A
- USB ports
- 2x USB-B 2.0 upstream (endpoint)
5x USB-A 2.0 downstream (of which 1 charge port)
- KVM switch
- Yes
- Power rating
- 24 VDC, 4 A
- Power requirements
- This device shall only be powered by the medical approved power supply of Adapter Technology Co., Ltd., type ATM160T-P240.
Ratings marked on the power supply:- Input: 100-240 VAC, 1.8-0.9 A, 50-60 Hz
- Output: 24 VDC, 6.6 A
- Power consumption
- 45 W (nominal)
< 0.35 W (hibernate)
< 0.30 W (switched off)
- Dimensions with stand (W x H x D)
- Portrait: 351 x 531~631 x 225 mm
Landscape: 491 x 462~562 x 225 mm
- Dimensions w/o stand (W x H x D)
- Portrait: 351 x 491 x 64 mm
Landscape: 491 x 351 x 64 mm
- Dimensions packaged (W x H x D)
- 455 x 210 x 770 mm
- Net weight with stand
- 8.8 kg
- Net weight w/o stand
- 5.8 kg
- Net weight packaged
- 12.2 kg (without optional accessories)
- Tilt
- -10° to +30°
- Swivel
- -30° to +30°
- Pivot
- 90°
- Height adjustment range
- 100 mm
- Mounting standard
- VESA (100 mm)
- Screen protection
- Protective, anti-reflective front glass
- Recommended modalities
- All digital images, except digital mammography
- Certifications
- CE0123 (Medical Device)
FDA 510(k) K230520
CCC (China), KC (Korea), BSMI (Taiwan), INMETRO (Brazil - Product numbers K9300390A1X, K9300391A1X), BIS (India), EAC (Russia, Kazakhstan, Belarus, Armenia and Kyrgyzstan)
Safety specific:- IEC 60950-1:2005+A1:2009+A2:2013
- EN 60950-1:2006+A1:2010+A11:2009+A12:2011+A2:2013
- IEC 62368-1:2018
- EN IEC 62368-1:2020+A11:2020
- IEC 60601-1:2005+A1:2012+A2:2020
- EN 60601-1:2006+A1:2013+A12:2014+A2:2021/li>
- AAMI ES 60601-1:2005+A1:2012+A2:2021
- CAN/CSA C22.2 No. 60601-1:2014 (Reaffirmed 2022)
- IEC 60601-1-2:2014+A1:2020 (Ed.4.1)
- EN 60601-1-2:2015+A1:2021 (Ed.4.1)
- FCC part 15 Class B
- ICES-001 Level B
- VCCI (Japan)
EU RoHS, China RoHS, REACH, Canada Health, WEEE, Packaging Directive
- Supplied accessories
- User Guide
- Documentation disc
- System sheet
- Video cables
- Mains cable(s)
- USB cable
- External power supply
- Optional accessories
- Display controller
- QA software
- QAWeb Enterprise
- Warranty
- 5 years, including 20000 hours backlight warranty
- Operating temperature
- 0 °C to 35 °C (20 °C to 30 °C within specs)
- Storage temperature
- -20 °C to 60 °C
- Operating humidity
- 8 % to 80 % (non-condensing)
- Storage humidity
- 5 % to 85 % (non-condensing)
- Operating pressure
- 70 kPa minimum
- Storage pressure
- 50 to 106 kPa
You can now find all media, brochures, presentations, whitepapers & marketing downloads in our new & improved download center
Visit Media CenterLooking for technical documents or product support?
For technical downloads such as drivers, firmware, manuals, drawings & documentation we would kindly like to direct you to our product support page.
Go to product support